Global trials are growing in scale, regulations are becoming stricter, and patient populations are more diverse than ever. For CROs, that means translation is no longer just part of trial administration; it’s now a strategic requirement for compliance and for ensuring that participants fully understand the purpose, procedures, and risks of the trial.
In this guide, we’ll walk you through the role of clinical trial translation services in modern clinical research, the challenges they address, and the best practices that ensure accuracy, compliance, and trust.
Table of Contents
Why Clinical Trial Translation Services Matter (The First Step to Compliance)
For clinical trials, compliance is the foundation of trust between sponsors, regulators, and patients. And at the center of compliance is one non-negotiable requirement, which is clear, accessible communication, an aspect that makes translation a regulatory mandate rather than being a support service.
To ensure compliance is more than just a principle, regulatory bodies have built translation requirements directly into their frameworks.
European Union: Clinical Trials Regulation (CTR)
The EU requires translations for both patient-facing and regulatory documents:
- For participants: All clinical trial materials must be translated into the language(s) spoken by trial participants to ensure informed consent and a clear understanding of rights, responsibilities, and risks.
- For regulators: Documents submitted to national authorities must be translated into the official language(s) of the member state hosting the trial.
The CTR also specifies that translations must be performed by qualified professionals, reviewed by subject-matter experts, and fully documented, including translator details and revision history.
United States: Food and Drug Administration (FDA)
The FDA places the same emphasis on clarity and accessibility. Its guidance requires that:
- Informed consent documents must be written and translated in a way that is clear, understandable, and culturally appropriate.
- Patient-facing materials must enable participants to fully grasp their rights, responsibilities, and potential risks.
United States: National Institutes of Health (NIH)
In 2024, the NIH strengthened its policies by mandating that informed consent documents be translated into the languages spoken by expected participants. It also allocated resources for:
- Translating study materials
- Hiring bilingual research staff
- Implementing culturally appropriate communication strategies
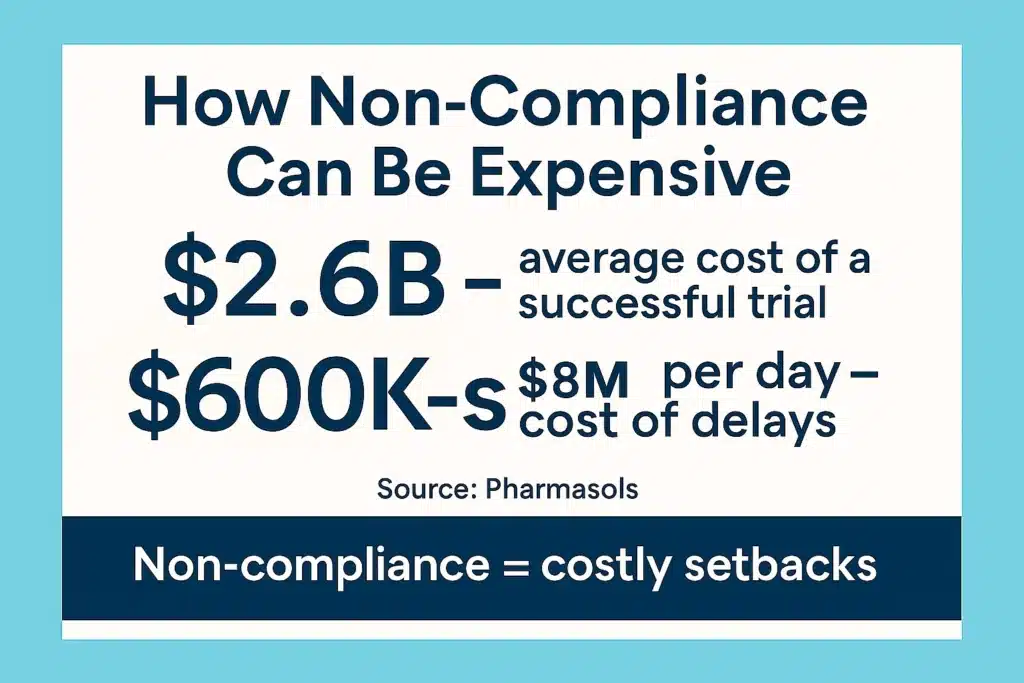
But while translation may start as a regulatory requirement, it actually shapes the entire patient journey, from enrollment to long-term participation.
The Language Factor in Patient Commitment
Clinical trials depend on diversity. To produce results that truly reflect real-world patients, you need participants from different racial, ethnic, and linguistic backgrounds.
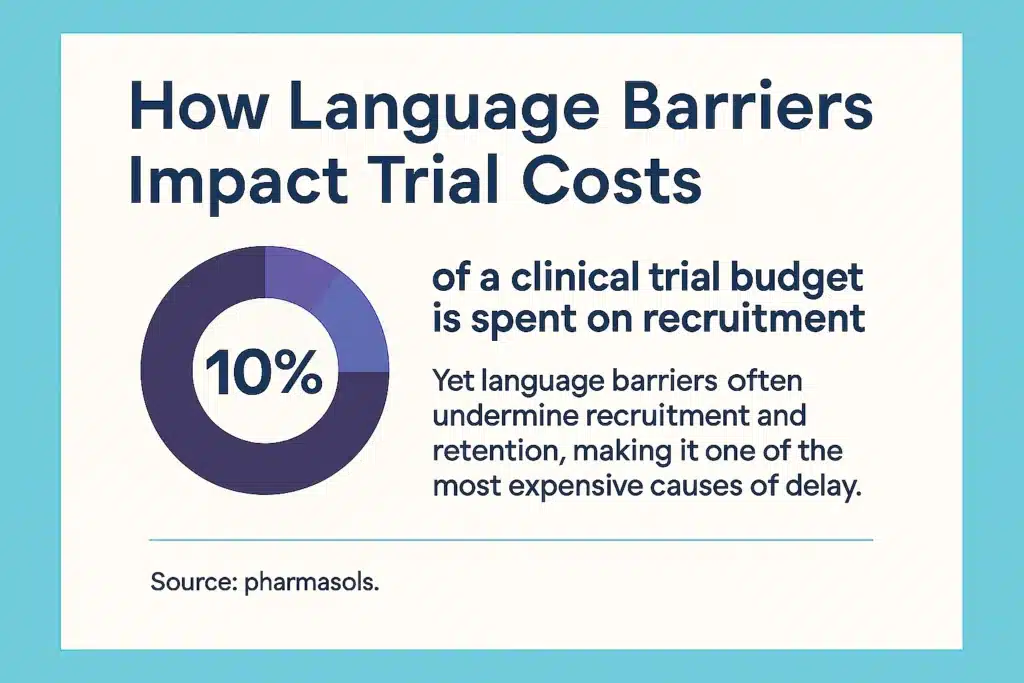
However, for Clinical Research Organizations (CROs), language barriers pose one of the biggest challenges to achieving this goal. These barriers complicate recruitment, retention, and communication, making it difficult to fully engage and support all patients throughout the trial.
Recruitment: Effective Participant Engagement
Recruitment is often the first point where language creates challenges. When trial information is unclear or not available in a patient’s primary language, potential participants are less likely to enroll.
Retention: Maintaining Engagement
Once enrolled, participants need to feel supported throughout the trial. If instructions are difficult to follow or communication with the research team is limited by language, participants may disengage, which increases dropout rates.
Continuity: Supporting Long-Term Participation
Even after enrollment, trials can face setbacks if participants do not fully understand instructions or cannot communicate their experiences in their own language. Successful trials depend on more than recruitment; they require participants to follow procedures, provide feedback, and complete their commitments.
The Scope of Modern Clinical Translation Services
Clinical trial translation services aren’t limited to a handful of forms. Every trial generates an interconnected system of documents that serve different audiences.
And each category requires a different balance of scientific accuracy, regulatory precision, and patient-friendly clarity, which makes the scope of clinical document translation both wide and complex.
Scientific documents (Mistakes Here Can Affect Study Design or Endpoints)
- Clinical Protocols
- Investigator Brochures
- Study Endpoints Documentation
- Pharmacovigilance Reports
Patient-facing materials (Errors in These Documents Can Compromise Consent and Ethics)
- Informed Consent Forms (ICFs)
- Patient Diaries & Questionnaires
- Patient Recruitment Materials (flyers, ads, websites)
- Patient Information Sheets
Regulatory submissions (Poor Translation of Regulatory Content Leads to Rejections or Delays)
- Ethics Committee Submissions
- Regulatory Authority Applications
- Safety Reports (SAEs/ SUSARs)
- Clinical Study Reports (CSRs)
Operational/training materials (Miscommunication Here Can Affect Trial Staff Performance)
- Case Report Forms (CRFs)
- Training Manuals & SOPs
- Site Instructions & Guidelines
- eLearning Modules for Investigators & Staff
When you multiply these document types across multiple countries, languages, and stakeholders, the challenge grows quickly. For CROs and sponsors, it’s not just about knowing what needs to be translated. The real challenge lies in managing the operational demands that come with this scope
The Challenges Behind the Scope
Managing Scale
A single Phase III trial can produce tens of thousands of pages across dozens of countries and languages. Translation isn’t about a few documents. It’s about coordinating a constant flow of sensitive materials under strict deadlines.
Maintaining Consistency
With so many documents developed in parallel, medical terminology must remain uniform. A drug name, dosage, or adverse event term must appear identically across protocols, CRFs, submissions, and patient-facing materials to avoid confusion, regulatory pushback, or safety risks.
Expert providers achieve this through advanced tools like translation memory, which stores and reuses approved terminology across all documents, ensuring accuracy, efficiency, and consistency at every stage of the trial.
Aligning Translation with Trial Workflows
Translation has to move in sync with regulatory submissions, site activation, and staff training. Any lag creates bottlenecks that ripple across the trial, delaying approvals, slowing recruitment, and disrupting the progress of clinical studies.
What Makes Truly High-Quality? The Pillars Defining Quality in Clinical Protocol Translation Services
In the context of global clinical trials, quality means more than clean grammar or accurate wording. In such a sensitive, high-stakes environment, translation becomes a matter of patient safety, data reliability, and regulatory compliance. That’s why understanding what truly defines quality and knowing what to expect from a translation partner is very important.
Below are the pillars that truly define high-quality clinical trial translation services and why they matter.
Medical Integrity
Medical integrity depends first and foremost on the expertise of the translators working on your project.
The provider you choose must assign your project to translators who specialize specifically in the therapeutic area involved, whether that’s oncology, cardiology, neurology, or any other medical field.
Their subject knowledge allows them to understand complex terminology, interpret context correctly, and preserve the integrity of every document.
If your partner lacks medical expertise, translations might:
- Misrepresent drug names, dosages, or adverse events.
- Distort the scientific meaning of endpoints or criteria.
- Fail to meet regulatory language standards, causing pushback or delays.
- Leave consent forms and patient materials unclear to participants.
- Misuse specialized abbreviations and acronyms in protocols and CRFs.
- Present safety information inaccurately, putting patients at risk.
- Create inconsistencies across trial documents, weakening data integrity.
- Undermine ethical standards, limiting patients’ ability to give informed consent.
Clarity
One of the first indicators of quality in clinical trial translation services is how well patient-facing materials can be understood.
Informed consent forms and other medical documents are often dense, legalistic, and full of technical language, and translating them word-for-word only makes them harder to read. True clarity means reworking those translations into plain, direct language that patients from different educational and cultural backgrounds can follow
The Patient-Facing Material Clarity Checklist
- Use plain, simple language
- Adapt to literacy levels
- Test readability and comprehension
- Keep sentences short and direct
- Avoid medical jargon unless essential
- Use culturally familiar examples or terms
- Ensure consistent terminology across materials
- Validate clarity through patient feedback
Cultural and Contextual Adaptation
Patients bring their own cultural expectations and health beliefs to the way they read and interpret information.
High-quality translation requires native-language medical experts who can adapt tone, phrasing, and examples to feel natural while preserving meaning. This balance is essential to building trust and supporting ethical transparency.
- Adjust for differences in directness, formality, and language preferences
- Avoid wording that could reinforce stigma and discourage diagnosis or treatment.
- Adapt how illnesses, symptoms, and treatments are described
- Replace idioms or culturally specific references with equivalents
- Ensure appropriate use of titles, honorifics, and pronouns
- Translate dates, times, measurements, and numbers into the locally accepted format
Version Control and Traceability
Clinical trials are constantly evolving. Protocols are amended, instructions updated, and materials reissued. Every change has to be reflected across all translated versions, often in dozens of languages, without delay or inconsistency.
LSPs equipped with advanced translation management systems and audit trails make this possible, giving you confidence that every update is captured, aligned, and fully traceable for regulatory review.
The Version Control Checklist
- Use a centralized TMS to track updates
- Keep audit trails of edits and approvals
- Sync updates across all language versions
- Maintain version logs for regulators
- Assign clear ownership of changes
- Validate updates against the latest source
Data Security
Trial documents contain sensitive patient information, proprietary study data, and regulatory submissions. A single breach doesn’t just put that information at risk; it can damage your reputation, compromise patient trust, and delay regulatory approvals.
That’s why true quality in clinical trial translation services means embedding confidentiality into every step of the process.
High-quality providers demonstrate this by:
- Signing strict NDAs that bind all parties involved
- Using encrypted workflows and file-sharing systems
- Applying role-based access controls to limit exposure
- Conducting regular security audits and risk assessments
- Storing data on secure, compliant cloud or on-premises systems
- Providing comprehensive staff training on data privacy protocols
- Maintaining immediate breach response plans to contain incidents
Quality Assurance and Linguistic Validation
The final pillar of clinical trial translation is about making sure nothing is left to chance. After all the effort that goes into accuracy, consistency, and cultural adaptation, this stage confirms that translations are not only error-free but also clear, compliant, and ready for both regulators and patients.
– Quality Assurance (QA)
- Multi-step reviews that include expert editing, proofreading, and back-translation
- Detects and corrects errors before delivery
- Confirms each translation meets the highest scientific and regulatory standards
–Linguistic Validation:
- Focuses on patient-facing materials like informed consent forms and questionnaires
- Verifies not only accuracy but also cultural and contextual appropriateness
- Uses cognitive debriefing with native speakers to confirm comprehension
- Ensures materials are clear, ethically sound, and support informed consent
The Bottom Line: Your Clinical Trials Translation Company Defines Your Outcome
Your language service partner is more than a vendor; they are a vital collaborator in your clinical trial journey. Choosing them is as important as selecting your research team because translation quality directly impacts patient safety, regulatory compliance, and the integrity of your data.
Selecting the right clinical trial translation services provider means choosing expertise, reliability, and commitment over convenience or cost alone. It means working with a team that understands your therapeutic area, safeguards your data, and scales with the demands of international clinical trials.
Investing the time to find a partner dedicated to your trial’s success is, ultimately, an investment in the success of the study itself





