Have a Big Project?
Big numbers, lots of documents and multiple translations?
Our top performing teams deliver unmatched quality on time for you
to hit your business goals.

Medical devices don’t move markets unless they move safely across borders. And when they do, it isn’t just about manufacturing or regulatory approval—it’s about language. The growth of global healthcare, from high-tech implants in Europe to diagnostic kits in Africa, has fueled unprecedented demand for medical device translation services.
In this article, we’ll unpack what medical device translation services really include, where demand is surging, the five key domains of medical device localization, how AI is accelerating the process, and the best practices that protect manufacturers from costly mistakes.
Medical device translation services refer to the structured process of adapting the technical, regulatory, and user-facing content into local languages so that devices can be legally approved, safely used, and effectively adopted in foreign markets.
Naturally, medical device translation services should ensure utmost linguistic accuracy. But the translation must also essentially be precise, consistent, and legally aligned with regulatory bodies such as the FDA (the USA.), EMA (EU), PMDA (Japan), SFDA (Saudi Arabia), and others.
A mistranslated line in a regulatory submission can delay approval for months. An inconsistent dosage unit on a label can trigger product recalls. This is why the industry treats medical device translation services as mission-critical infrastructure.
The demand for medical translation services is driven by the medical device industry’s global growth.
The global medical devices market size was valued at USD 640.45 billion in 2024 and is projected to reach USD 678.88 billion in 2025. Looking ahead, the market is expected to expand steadily, reaching approximately USD 1,146.95 billion by 2034, at a compound annual growth rate (CAGR) of 6.% during the forecast period.
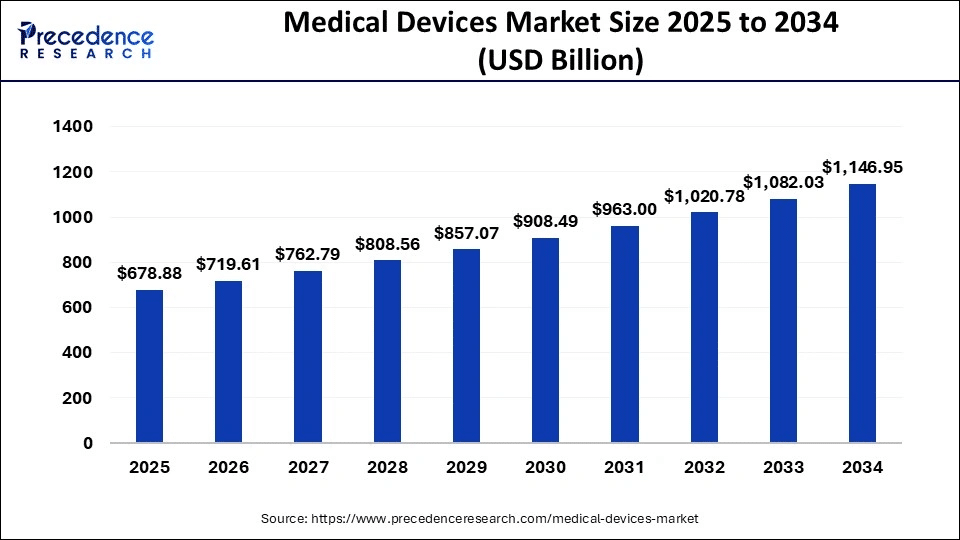
Devices crossing borders at this pace require regulatory approval in dozens of jurisdictions, accurate labeling in multiple local languages, and consistent adaptation of user interfaces for doctors and patients worldwide.
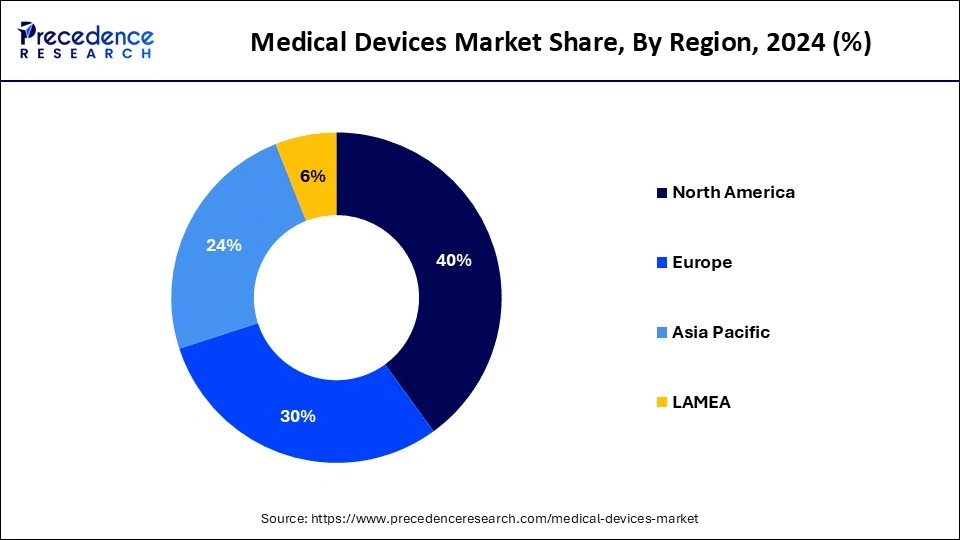
The Middle East and North Africa (MENA) region is a fast-emerging market. As reported by industry experts, Egypt, Morocco, and Saudi Arabia are actively strengthening medical device regulations, with localization at the center, pushing forward with reforms that make medical device registration more accessible but also more localized.
Egypt’s Health Authority, for example, increasingly demands documentation and user manuals in Arabic to ensure clinician and patient safety. Saudi Arabia’s Saudi Food and Drug Authority (SFDA) aligns with international standards but requires all labeling and IFUs in Arabic for approval.
For manufacturers targeting the Gulf and North Africa, medical device translation services are no longer a competitive advantage—they are a market entry requirement. This region alone represents billions in healthcare investment, and linguistic adaptation is the ticket in.
When companies discuss medical equipment translation, they often think only of IFUs or labels. In reality, the scope is far broader. Below are the five essential pillars where translation and localization shape compliance, usability, and market access.
The foundation of medical device translation services lies in regulatory paperwork. Devices cannot enter a market without complete medical device documentation translation that meets the standards of local regulatory bodies. This includes:
Regulators scrutinize these for consistency, and quality assurance processes require language precision. A device manufacturer may spend millions on clinical trials, but if translations of trial results or adverse event reports are inaccurate, the entire submission risks rejection.
Labels carry more than product names—they carry patient safety.
Medical device translation services here include everything from dosage and measurements to sterility warnings, expiration dates, and unique device identification (UDI). The localization process must also account for country-specific UDI requirements and pictograms.
Errors here are not just regulatory issues. They are patient safety failures. A mistranslated dosage could mean an overdose. That’s why labeling translation demands both linguistic accuracy and regulatory expertise.
IFUs and user manuals bridge the gap between technology and the human beings operating it.
For medical device manufacturers, this means ensuring consistent translation of installation guides, operation manuals, and training materials for clinicians and technicians. Digital guides, firmware instructions, and even video training content fall under this umbrella.
The EU MDR is explicit: IFUs must be translated into the official language(s) of every market where the device is distributed. That’s not optional. It’s the law.
The scale is daunting — a device launched across the EU may require 20+ versions of its IFU. But consistency here is as much about safety as it is about law. Non-compliance leads to immediate rejection.
Medical devices are increasingly digital. From touchscreens on diagnostic machines to mobile apps connected to wearable devices, user interfaces require careful adaptation.
Medica device translation services become more than translating text-based content to convey meaning. It includes localizing user interfaces, medical units of measurement, date formats, and error messages. For example, a blood glucose meter with mmol/L in Europe must show mg/dL in the U.S.
Localization of digital interfaces must therefore balance linguistic adaptation, technical accuracy, and compliance with regulatory bodies that audit these systems.
Finally, medical devices don’t sell themselves.
That means localized marketing materials, from brochures and websites to patient education leaflets. These materials are often underestimated, yet they directly impact trust, adoption, and compliance. For example:
And because medical advertising is tightly regulated, translations must balance persuasion with compliance. The commercial success of a device in global markets depends on how well companies communicate its value, and that requires culturally adapted, accurate translation of every commercial asset. A single mistranslation in a promotional claim can trigger regulatory fines and reputational damage.

For decades, the medical device localization process for medical devices was notoriously slow. Regulatory submissions moved slowly through multiple human review cycles. IFUs took months to translate into dozens of local languages. Inconsistencies across documents triggered costly delays.
For manufacturers racing to meet launch deadlines, every delay equals lost revenue and delayed patient access.
That reality is changing. Technology — and specifically AI — is recalibrating how medical device translation services are executed, reviewed, and deployed at scale. But the story isn’t as simple as “machines replacing humans.”
In medical devices, the stakes are too high, the regulations too strict, and the linguistic, cultural, and regulatory requirements too complex for AI to act alone. Instead, the future is hybrid: automation for speed and volume, human experts for accuracy and compliance.
AI has already proven it can reduce cost and time-to-market for device companies. Machine translation engines trained on large medical corpora are capable of pre-translating regulatory dossiers, IFUs, and regulatory documents with remarkable speed. What used to take weeks of manual effort can now be delivered in days.
AI’s role isn’t limited to text. Medical device companies are increasingly embedding AI into workflow automation:
The result isn’t just faster content production. It’s a more scalable infrastructure that allows manufacturers to manage localization across 20, 30, or even 50 markets without collapsing under administrative burden.
The Future Model: Hybrid Localization
There’s another side to every story.
AI is powerful, but it cannot guarantee high-quality medical device translation on its own. The consequences of error are too severe.
This is why every AI-assisted workflow in the medical device space still relies on human oversight. Translators, reviewers, regulatory experts, and in-country specialists ensure that what machines accelerate is still compliant, accurate, and culturally aligned.
Companies entering global markets often underestimate the complexity of medical device translation services. It’s not a side task handled at the end of development. It’s a core discipline that shapes regulatory approval, market access, and patient safety. Here are the best practices that consistently separate successful launches from costly setbacks.
Every market writes its own rules, and many don’t align.
The risk lies in underestimating the differences. A submission that passes in Germany could fail in Saudi Arabia simply because Arabic instructions are missing. The solution is to work with localization services providers who maintain in-country regulatory specialists. These experts monitor changes, interpret ambiguities, and ensure that translations align with both global frameworks (ISO, CE, FDA) and local expectations.
Non-compliance here isn’t just about delays. It can mean outright exclusion from billion-dollar markets.
Medical device manufacturers require more than language skills. They need teams certified under ISO 17100 for translation quality and ISO 9001 for operational consistency. But certifications are just the start. A true provider fields subject matter experts across disciplines:
AI and automation are infrastructure. From machine translation engines to NLP-driven quality assurance processes, technology accelerates work that once stalled global launches. But the winning formula is hybrid:
Together, they deliver translations that are faster, cheaper, and more consistent — but still trusted by regulatory bodies worldwide.
Too many manufacturers treat localization as a compliance checkbox. In reality, it is a direct driver of user experience and patient safety.
A ventilator with a poorly translated error alert isn’t just a usability issue. It’s a risk. A home-use device with an ambiguous IFU isn’t just inconvenient. It’s dangerous. Standards like IEC 62366-1 make usability engineering a regulatory requirement, not a suggestion.
Prioritizing localizing medical content for clarity and accessibility isn’t just about compliance — it’s about building trust with clinicians and protecting patients.
Quality assurance is the invisible scaffolding that holds the entire process together. The most effective programs build around five pillars:
Golden Rule: In medical device translation, precision saves more than costs — it safeguards lives and protects your market access.
The future of medical device translation services is clear: it’s faster, smarter, and more global than ever. But speed without compliance is dangerous. And compliance without cultural and linguistic accuracy is incomplete. Medical device companies that treat translation as a strategic investment—not an afterthought—will be the ones expanding confidently into global markets.
From regulatory dossiers to user manuals, from regulatory bodies to patient safety, the mandate is simple: accurate, compliant, and culturally attuned translation isn’t negotiable. It’s the infrastructure that makes global healthcare possible.
The opportunity is massive. The responsibility is greater. And the companies that get it right won’t just access new markets—they’ll build trust, safeguard patients, and shape the future of medical technology worldwide.
Big numbers, lots of documents and multiple translations?
Our top performing teams deliver unmatched quality on time for you
to hit your business goals.
Ready to burst your borders and need work done quickly?
Order Now! To get an instant assessment and quote.
Don’t wait, we’re ready.
SAVE TIME & MONEY!
Order from our mobile app now